Background: Clinical outcome of patients (pts) with diffuse large B-cell lymphoma (DLBCL) has improved during the last two decades. However, a significant proportion of DLBCL pts is still at risk of lymphoma relapse (REL)/progression or death due to progression. DLBCL pts with adverse clinical features, i.e., those with high IPI or CNS IPI scores are at the highest risk of lymphoma REL, systemic (S) or central nervous system (CNS) REL. CNS prophylaxis is questionable based on retrospective analyses. Moreover, the risk of S-REL should be discussed in those pts as well. However, the comparison of the risks of CNS vs S-REL within the different CNS IPI risk groups has not been shown.
Methods: Using the prospective observational NiHiL project (NCT03199066), we identified pts ≥ 18 years of age with histologically confirmed DLBCL or high-grade B-cell lymphoma (diagnosed 2011-2021), who received R-CHOP as frontline treatment in 2 centres in Czech Republic. Pts with CNS involvement at diagnosis were excluded. Cumulative incidence of CNS (± S) REL was calculated using Kaplan-Meier statistics treating S-REL and death as competing events.
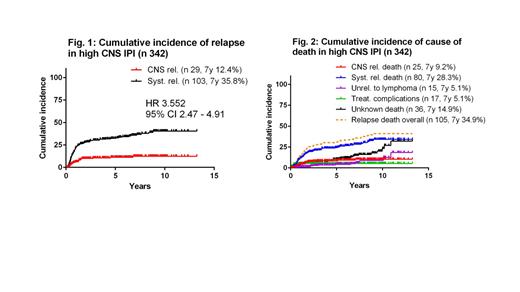
Results: A total of 1242 pts were included; according to CNS IPI score (available in 1225 out of 1242 pts), 344 (28.1%), 539 (44.0%) and 342 (27.9%) pts were categorized as being at low (0-1, LR), intermediate (2-3, IR), and high risk (4-6, HiR) resp. Overall, 308 (24.8%) pts received CNS prophylaxis with either high-dose(HD) Methotrexate (MTX), HD-AraC, intrathecal chemotherapy or combination. By CNS IPI, 150 (43.9%) out of 342 pts with HiR CNS IPI received CNS prophylaxis. CNS prophylaxis was given less frequently in the IR (n 111[20.6%] out of 539 pts) or LR (n 45 [13.1%] out of 344 pts) CNS IPI groups. At a median follow up of 5.3 years (range; 0.1 - 13.3), 307 (24.7%) out of 1242 pts developed lymphoma REL, out of which 45 occurred in CNS (35 isolated CNS REL, 10 simultaneous CNS+S-REL) and 262 lymphoma RELs occurred systemically. CNS prophylaxis had no impact on the incidence of CNS REL in pts with HiR CNS IPI. Median time to CNS (± S) and S-REL was 11.5 months (range; 2.7-116.8) and 11.8 months (range; 0.8 - 144.3), resp. Occurrence of RELs within the first 6, 12 and 24 months after lymphoma diagnosis was as follows: 20%, 53.3% and 80% for CNS (± S) RELs and 21%, 50.4% and 67.2% for S-RELs. In the entire cohort, the 7-year cumulative incidence of CNS (± S) and S-REL were 4.4% and 23.7%, resp. Across all CNS IPI risk groups, there was significantly higher risk of S-REL as compared to CNS (± S) REL. The 7-year cumulative incidence of S-REL vs CNS (± S) REL and the respective HRs were 10.5% vs 1.9%, HR 6.2 (95% CI 3.4-11.2) in the LR-, 25.2% vs 2%, HR 11.7 (95% CI 8.3-16.6) in the IR-, and 35.8% vs. 12.4 %, HR 3.6 (95% CI 2.5-4.9) in the HiR CNS IPI ( Fig1), resp. At the time of analysis, 417 (33.6%) out of 1242 pts were dead. CNS (± S) REL was associated with shorter post-REL overall survival (OS) as compared to S-REL (HR 1.8, p=0.001, median OS 0.38 vs 1.04 years, resp). In the entire cohort, the 7-year cumulative incidence of death due to lymphoma was 19.3% (16.7% and 3.1% due to S-REL and CNS (± S) REL, resp). The 7-year cumulative incidence of death due to R-CHOP-related toxicity, unrelated to lymphoma and unknown causes were 2.5%, 4.2% and 9.8%, resp. In HiR CNS IPI, the 7-year cumulative incidence of death due to lymphoma was 34.9% (28.3% and 9.2% due to S-REL and CNS (± S) REL, resp), while the 7-year cumulative incidence of death due to R-CHOP-related toxicity, unrelated to lymphoma and unknown causes were 5.1%, 5.1% and 14.9%, resp ( Fig2).
Conclusion: DLBCL pts are at significantly higher risk of S-REL as compared to CNS (± S) REL overall and across all CNS IPI risk groups. In addition, death due to S-REL is the leading cause of death overall and across all CNS IPI risk groups. However, CNS (± S) REL represents remarkable portion of all lymphoma RELs occurring in the HiR CNS IPI group and non-negligible cause of death in DLBCL. Our results support the conclusion that R-CHOP + HD MTX is not sufficient to reduce the risk of S-REL as well as CNS REL especially in pts with HiR CNS IPI. New 1 st line treatment options able to reduce simultaneously the risk of S-REL as well as CNS REL are needed to improve the outcome of DLBCL pts especially in the subgroup with HiR CNS IPI.
Grant support: AZV NU23-03-00127, NU21-03-00411, Charles University Hematology-Oncology Cooperatio Program
Disclosures
Klanova:Tubulis: Ended employment in the past 24 months. Vodicka:Roche: Consultancy. Janikova:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Trneny:Takeda, Bristol-Myers Squibb, Incyte, Abbvie, Amgen, Roche, Gilead Sciences, Janssen, MorphoSys, Novartis, Genmab, SOBI: Consultancy; Janssen, Gilead Sciences, Takeda, Bristol-Myers Squibb, Amgen, Abbvie, Roche, MorphoSys, Novartis: Honoraria; Gilead Sciences, Takeda, Bristol-Myers Squibb, Roche, Janssen, Abbvie: Other: Travel, Accommodation, Expenses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal